

The AAOS Registry Analytics Institute® (RAI) is a resource to the scientific community, created to improve orthopaedic and musculoskeletal care by making data analyses available.
Investigators can submit hypotheses about information in AAOS registries.
AAOS committees provide peer review process and oversight to which proposals are approved. Data analysis will be completed by AAOS Registry Analytics team members for all approved proposals.
Application Process
To participate in data analysis collaboration through the RAI, please complete the web-based RAI application. Potential applicants are strongly advised to review the AAOS American Joint Replacement Registry (AJRR) data elements prior to applying to understand what data is available for study.
Eligibility for Analysis Requests
Analysis requests are open to investigators with a well-defined hypothesis or research question related to orthopaedics or musculoskeletal care.
For this initiative, “investigators” are defined as clinicians or clinician-scientists affiliated with a clinical practice or care setting. The following entities are not eligible to apply:
- Representatives from industry, federal agencies, or commercial entities
- Individuals affiliated with insurance companies, administrative databases, or hospital consortia
Application Limits
- Each applicant may submit up to two (2) applications per cycle, with a maximum of four (4) applications per year.
- Each institution is limited to four (4) applications per cycle, with a total of eight (8) applications per year.
If an RAI application is accepted for analysis, no additional applications will be considered from the same investigator until the results of the ongoing RAI project are submitted for peer-reviewed publication or presented at a conference. This policy ensures the timely dissemination of findings before new applications are reviewed.
Request Process
The RAI cycle is a rolling application process, allowing potential applicants to apply year-round. Applications submitted before the Cycle 1 deadline will be reviewed during Cycle 1, while those submitted after the Cycle 1 deadline but before the Cycle 2 deadline will be considered in Cycle 2.
| Season Cycle | Application Deadline | Feasibility Assessments Completed | Grading Completed and Applicants Notified |
| Cycle 1 (Annual) | March 1 | March 15 | April 1 |
| Cycle 2 (Annual) | September 1 | September 15 | October 1 |
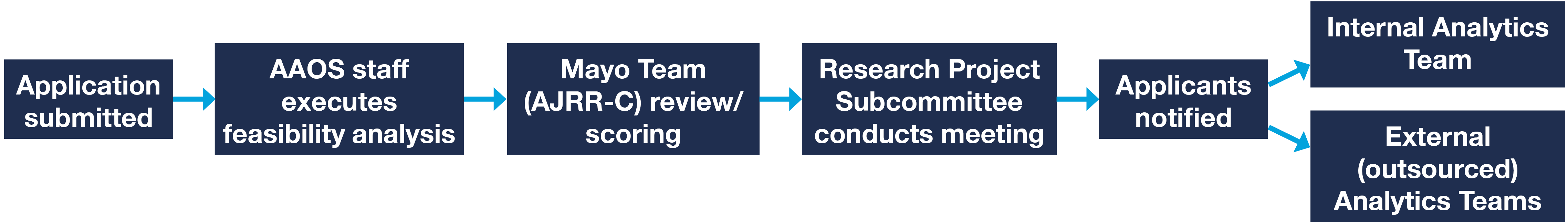
Applications can be submitted until 7 p.m. (Central Time) on the day of the deadline. Submissions received after the deadline will be deferred to the next review cycle. When an application is submitted, the proposed project enters the AAOS Analyses Request and Publications pipeline and proceeds along the process shown below. Analyses for all applications will be completed and sent back to the submitter within a year of application approval. AAOS staff will try and accommodate abstract deadlines and urge submitters to apply as soon as possible to increase the likelihood of achieving desired timelines.
Application Review, Summary Flow Chart
Available Data
The AJRR collects data related to procedures and post-operative care. AJRR data is linked to Medicare data to ensure capture of all inpatient and outpatient cases for patients above 65. Therefore, AAOS analysts may suggest limiting study populations to ensure accurate follow-up. View an overview of AJRR data elements.
Data Analysis
Approved projects will work with the AAOS Registry Analytics team to develop an analysis plan and timeline. At the completion of the project, investigators will receive a completed, de-identified analysis.
Post-Acceptance Process
For details on the next steps after acceptance, please refer to the RAI Process for Accepted Applicants.
See a list of papers and presentations based off RAI completed applications.
Publication Policies
Scientific Publications
Recipient shall ensure that all proposed scientific publications (including manuscripts and abstracts for presentations) arising from the use of the analyses provided pursuant to this Agreement are submitted to AAOS for review and comment at least one month prior to submission for publication or making public any information that has been derived utilizing the Registry Reports. Any reasonable amendments requested by the AAOS with regards to its analyses will be incorporated. The AAOS reserves the right of veto.
If AAOS staff or volunteer leaders collaborate with Recipient on a scientific publication, such individual or individuals will be listed as a co-author in accordance with guidelines issued by the International Committee of Medical Journal Editors. Recipient agrees to acknowledge the contribution made by the AAOS in all publishable work arising from the analysis and will provide copies of such publications, presentations and abstracts to be placed on the AAOS website. The acknowledgement must include the following wording:
“This article is based [in whole] [in part] on reports and/or analyses from the American Academy of Orthopaedic Surgeons’ American Joint Replacement Registry (AAOS). AAOS is not responsible for and does not necessarily endorse the analysis or conclusions in this article.”
Commercial/Promotional Claims
Any public commercial/promotional claims or statements, whether issued in electronic (written, audio or audiovisual) or print format, made by Recipient that are based on or are derived from the Registry Reports shall be complete and accurate excerpts from finalized publications that have been subject to the process set forth in Section 2(b) herein. Such statements also must include a legend or footnote in conspicuous size and type that reads as follows:
“The American Academy of Orthopaedic Surgeons (AAOS) is not responsible for the claims made in this [advertisement] [statement]. AAOS does not endorse companies or their products.”