Compared with the United Kingdom National Joint Registry (NJR), hip and knee arthroplasty device-specific survivorship results in the American Joint Replacement Registry (AJRR) were consistent and showed similar trends for comparatively high- and low-performing devices through final follow-up at 8 years. These findings were reported in a study performed in collaboration with the Orthopaedic Data Evaluation Panel (ODEP), an independent panel that reviews implants as they come to market and as they mature.
Three primary total knee arthroplasty (TKA) devices and three primary total hip arthroplasty (THA) devices were evaluated. They were selected by AJRR and ODEP and had known variations in performance and design. The study covered 42,671 AJRR and 60,439 NJR primary knee cases and 70,169 AJRR and 422,657 NJR primary hip cases. The implant manufacturers independently produced results of Kaplan-Meier survivorship using NJR data. The AJRR mirrored the methodology, and results from both sources were stratified into three age cohorts (all-age, <65, and ≥65 years).>
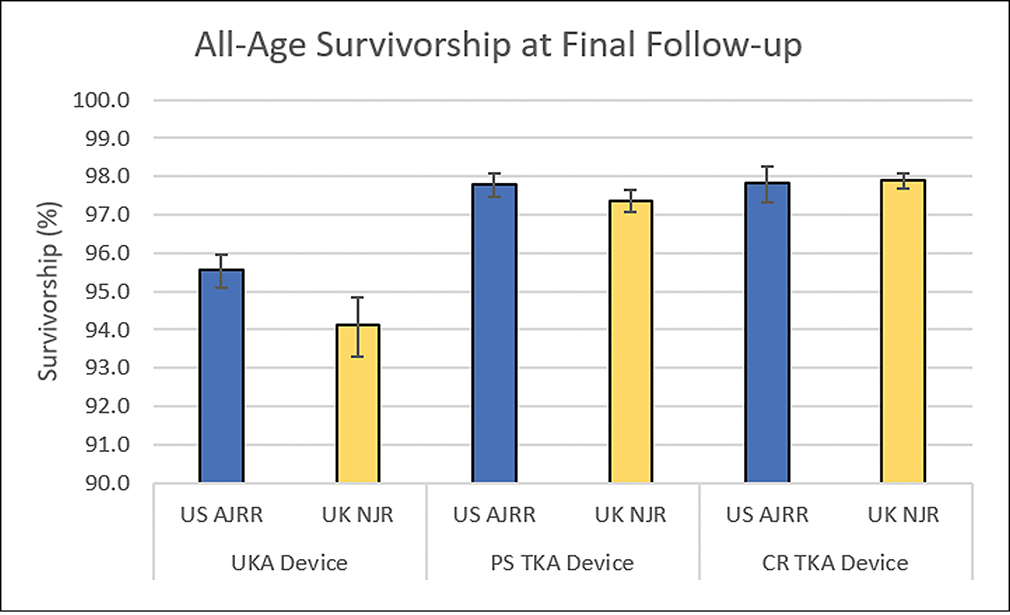
For TKA, the three device types were posterior stabilized (PS), cruciate retaining (CR), and unicompartmental knee arthroplasty (UKA). Performance between AJRR and NJR was consistent through the 8 years of follow-up, and both PS and CR devices showed statistical agreement in survivorship for all three age cohorts.
The UKA comparison also showed statistical agreement for the ≥65 years and <65 years cohorts. the all-age cohort showed similar trends and reached statistical agreement through 7 years. overall, ajrr and njr performance trends and survivorship were similar across knee arthroplasty devices, with greatest consistency in the all-age and age ≥65 cohorts.>
For THA, performance between AJRR and NJR were consistent, showing similar trends for comparatively high- and low-performing devices, with an 0.18 percent average difference between registries in survivorship at 8 years. The hip components examined were cementless acetabular socket, cementless fit and fill hip stem, and cementless blade-type hip stem. One femoral device did not reach statistical agreement but showed only 0.61 percent difference in survivorship at final follow-up. The remaining acetabular and femoral devices reached statistical agreement in the all-ages cohort and through 7 and 8 years in the ≥65 years cohort.
Validating AJRR outcomes
Explaining the rationale for the study, Bryan D. Springer, MD, FAAOS, first author and the 2020–2022 chair of the AJRR Steering Committee, noted that AJRR currently has 1,500 institutions and 13,000 registered surgeons submitting data, making it procedurally one of the largest total joint replacement registries in the world.
“But as many people know, we don’t capture 100 percent of all joint replacements that are done in the United States, with the numbers somewhere around 42 to 45 percent of all total joints done here,” he noted.
That capture rate contrasts with a number of mature registries around the world that have a 90 percent to 100 percent capture rate. “Because of that relatively low capture rate, the question always is, ‘Are the data representative of what’s going on across the United States and around the world?’ particularly if you’re going to use it for comparative purposes or for research,” he said.
AJRR previously published two studies looking at “data representativeness in the United States” in comparisons with other national registries. These studies found that data were indeed representative of what is being done in total joint replacements across the U.S. population. “The bigger question then becomes, are the data in the AJRR representative of what we’re seeing worldwide with these registries that have huge capture rates?” Dr. Springer said. “That has pretty significant implications when we start talking about doing comparisons of what’s going on in the United States versus in other countries that have large registries.”
These comparisons have significance for attaining regulatory approval of implants internationally. More implant models are entering the U.S. market than anywhere else in the world, and international regulators look to the ODEP ratings. According to Dr. Springer, in order to use AJRR data to be able to get ODEP ratings, “We have to make sure that our data are consistent with what’s being reported in some of the other international registries.” The resulting study, he said, was the first device-specific comparison of AJRR data with international registry data.
The study stratified results on hip and knee devices by age, looking at patients younger than 65 and those 65 and older, because, as Dr. Springer described, “In AJRR, we capture 100 percent of patients who are in the Medicare population because we have access to all that data. But we potentially lose out on some patients who are younger than 65 if, for example, they have their joint revised at a hospital that does not report to AJRR.”
The main takeaway, he said: “For all three devices, there was very good statistical agreement; we determined that by having overlapping confidence intervals for each of the devices at various years.” The team assessed confidence intervals for years 1, 3, 5, 7, and 8.
Even in areas where there were some minor statistical disagreements, the overall difference in survivorship was small. “Those comparisons really lead us to conclude that the performance of knee implants in the AJRR were very similar to what we’re seeing in a large-scale registry, across all age groups, across all cohorts,” Dr. Springer said.
Benefits of data alignment
Specifically, the findings yield two major benefits. “One, they obviously validate that our data not only represent a national representative sample,” Dr. Springer explained. “That has implications for global improvement in patient safety. What we’re most excited about is that this has led to pilot programs with ODEP, by which they allow the AJRR data to be used to get ODEP ratings.”
This development has positive implications both for validation of the AJRR and for industry partners in the registry. “These manufacturers now can use AJRR data and submit those data to ODEP to get ratings on their implants instead of having to do a lot of this work internally, which is expensive,” he said.
“We’re extremely excited about this pilot, and we hope that it’s going to continue to be expanded,” Dr. Springer said.
Ultimately, he said, he anticipates that the use of joint replacement registry data for ODEP ratings will become standard across the hip and knee arthroplasties and eventually across other devices.
Dr. Springer’s coauthors of “Is the American Joint Replacement Registry Data Consistent with International Survivorship in Hip and Knee Arthroplasty? A Comparative Analysis” are James I. Huddleston, MD; Kyle Mullen, MPH; Patrick Donnelly, MA; Edward Caton; and Keith Tucker.
Terry Stanton is the senior medical writer for AAOS Now.