Editor’s note: This article is part three in a three-part series on osteobiologics. Part one, published in the November issue, discussed regulatory pathways of osteobiologics. Part two, published in the December issue, provided an explanation of how osteobiologic products are categorized.
The use of osteobiologics in orthopaedics is increasing rapidly. With more than 350 osteobiologic options available today, clinicians often face contradictory claims and evidence, as well as significant variations in cost, which confound value-based treatment decisions.

^ HCT/Ps are eligible for regulation exemption under Section 361 as minimally manipulated products where the mechanism of action is not “dependent on the metabolic activity of living cells,” otherwise they would be regulated as Class III medical devices.
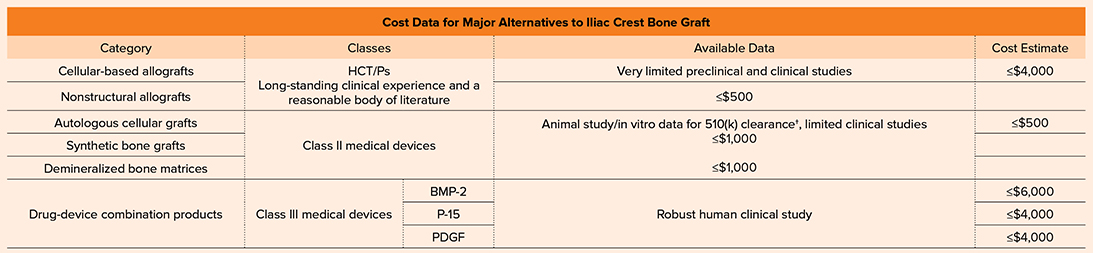
Table 2 Available data and cost estimate of major alternatives to iliac crest bone graft. Cost estimates are based on U.S.-based cost information; costs vary according to location, product volume, and procedure. HCT/P, human cell or tissue product; BMP, bone morphogenetic protein; PDGF, platelet-derived growth factor
According to Karl Roberts, MD, FAAOS, FAOA, chair of the AAOS Evidence-Based Quality and Value Committee, “One of the strategic initiatives of AAOS is to equip members with the tools necessary to thrive in value-based environments and advance the quality of orthopaedic care. While there is considerable opportunity in the world of biologics to advance the quality of care for our patients, the rapid evolution of these technologies has led to variability in products, cost, and outcomes. Clinical practice guidelines (CPGs) are a great resource to help guide our fellowship with respect to evidence-based recommendations; however, the feasibility of a powerful CPG is limited without a sound scientific foundation of evidence. As additional research is done to validate the efficacy of osteobiologic treatments, CPGs will be more useful to help guide clinicians and inform patients about indications and appropriate use.”
Biomechanical factors and clinical evidence
As a first principle, clinicians should have an appreciation of the healing environment and the physiological and biomechanical factors affecting bone regeneration, such as anatomical location, defect size, pathology, systemic and local biology, invasiveness of the procedure, and degree of mechanical stability. It is important for clinicians to recognize that there are variations in the performance of osteobiologics. Evidence of regenerative success and safety in one environment may not translate directly to another.
Different indications require bone-graft products that support new bone formation tailored to specific clinical settings. Surgeons often customize the use of bone-graft components to improve regenerative potential, aid in handling, or add graft volume. This leads to significant clinical variations in the use of osteobiologics (e.g., idiosyncratic formulations of mixing and dosage). These idiosyncratic therapies make it difficult to identify clinical effects and assess product value.
Physiological and biomechanical factors aside, the next guiding principle is to choose an osteobiologic based on the level of supporting evidence. Finally, that should be balanced against cost-effectiveness. Osteobiologics have the potential to significantly advance the standard of orthopaedic care; however, most osteobiologic options enter the market with little or no clinical evidence, making it difficult to predict effectiveness and safety. There is currently a paucity of high-quality human studies for osteobiologics. This makes it difficult for orthopaedic surgeons to use clinical evidence based on robust human clinical studies for decision making on osteobiologic utilization.
Evaluating available data
There is currently no universally adopted standardized approach to define strength of outcomes. Different societies and groups rely on different criteria. It is therefore useful to focus on study types and which provide the highest-quality evidence.
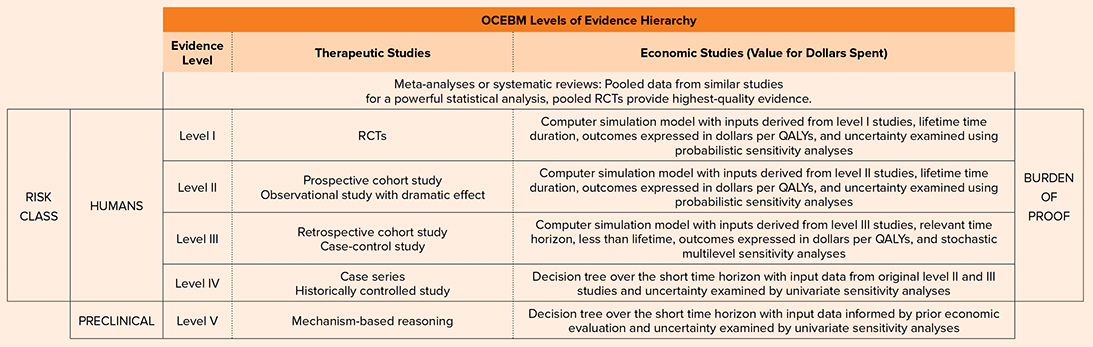
As summarized in parts one and two in this series, the level of evidence available for a product is often defined by FDA classification and the associated FDA regulatory pathway. A higher-risk class typically requires a greater burden of proof and regulatory scrutiny. A useful overview of evidence hierarchy based on the Oxford Centre for Evidence-Based Medicine (OCEBM) is presented in Table 1.
Orthopaedic surgeons can use the hierarchy to select products, identifying which products have high-quality evidence demonstrating safety and efficacy in humans. The vast majority of orthopaedic bone-graft products currently on the market are regulated via pathways that do not require studies in humans. The products that have the most rigorous clinical studies, have the highest burden of proof, and are subjected to the greatest regulatory scrutiny (typically Class III devices) also have the most restrictive on-label indications.
Assessing cost
Physicians and other healthcare workers are under increasing pressure to manage costs and justify the clinical value of new and existing technologies. Although numerous studies report on the regenerative potential and safety of osteobiologics, cost-effectiveness data are limited. These types of analyses are limited by lack of high-quality effectiveness data, heterogeneity in study design and product use, and transparency regarding product and procedure costs.
Future research initiatives are likely to address this gap, but for now orthopaedic surgeons must continue to rely upon critical evaluation of the available evidence to support decision making. Table 2 summarizes the available data per product category and estimates of cost.
Orthopaedic surgeons are key decision-makers for product selection, including osteobiologics. This is not an easy task in a saturated market where many biologic products have a lack of or low levels of clinical evidence in humans. It is important to be aware of the level of evidence available on a particular product, as well as clinical setting, when selecting an osteobiologic. This will drive evidence-based decision making and support the use of bone-graft products that provide clinical value, ensuring patient safety and efficacy while advancing the standard of orthopaedic care.
The AAOS Biologics Dashboard is an online tool designed to help orthopaedic surgeons navigate the approval status of biologic-based interventions. It combines the most recent and readily available clinical evidence with guidance released by the FDA and will stay current as osteobiologic therapies evolve. AAOS also published the first edition of the Orthobiologics textbook in February 2023, which is an excellent reference on biologics in orthopaedics.
John Cherf, MD, MPH, MBA, FAAOS, is a member of the AAOS Board of Councilors, member of the AAOS Evidence-Based Quality and Value Committee, and vice chair of the Board of Councilors Committee on Economic Issues. Dr. Cherf is the immediate past president and board member of the Illinois Association of Orthopaedic Surgeons and a member of the Buehler Center for Health Policy and Economics at Northwestern University Feinberg School of Medicine in Chicago.
References
- OrthoInfo: Orthopaedic Evidence-Based Medicine. Available at: https://orthoinfo.aaos.org/en/treatment/orthopaedic-evidence-based-medicine. Accessed Aug. 23, 2023.
- Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Joint Surg Am 2015;97(1):1-2.
- Abjornson C, Brecevich A, Callanan T, et al: ISASS recommendations and coverage criteria for bone graft substitutes used in spinal surgery. Int J Spine Surg 2018;12(6):757-71.
- Dubin JR, Simon SD, Norrell K, et al: Risk of recall among medical devices undergoing US Food and Drug Administration 510(k) clearance and premarket approval, 2008–2017. JAMA Netw Open 2021;4:e217274.
- Greenwald AS, Boden SD, Goldberg VM, et al: Bone-graft Substitutes: Facts, Fictions & Applications. Available at: https://orl-inc.com/wp-content/uploads/2007/03/Bone-Graft-Substitutes-Facts-Fictions-Applications-2007.pdf. Accessed Aug. 23, 2023.
- Demetriades AK, Mavrovounis G, Deml MC, et al; AO Spine Knowledge Forum Degenerative: What is the evidence surrounding the cost-effectiveness of osteobiologic use in ACDF surgery? A systematic review of the literature. Global Spine J 2023:21925682221148139.
- Niedermeier SR, Apostel A, Bhatia S, et al: Cost estimates of biologic implants among orthopedic surgeons. Am J Orthop (Belle Mead NJ) 2014;43(1):25-8.
- Hsieh PC, Buser Z, Skelly AC, et al. Allogenic stem cells in spinal fusion: a systematic review. Global Spine J 2019;9(1 Suppl):22S-38S.
- Boden SD: Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine (Phila Pa 1976) 2002;27(16 Suppl 1):S26-31.
- Tavares WM, de França SA, Paiva WS, et al: A systematic review and meta-analysis of fusion rate enhancements and bone graft options for spine surgery. Sci Rep 2022;12(1):7546.
- Cannada LK: Viable bone and circulatory factors required for survival of bone grafts. Orthop Clin North Am 2010;41(1):5-13.
- Smith KA, Russo GS, Vaccaro AR, et al: Scientific, clinical, regulatory, and economic aspects of choosing bone graft/biological options in spine surgery. Neurosurgery 2019;84(4):827-35.