Ultrasonographic-Guided Injection and “Pie Crust” Technique of Capsular-Tendon Structures for Treatment of Symptomatic Bipartite Patella
Introduction
Initial management of a painful bipartite patella is nonsurgical and includes restriction of activities, immobilization and bracing, administration of nonsteroidal anti-inflammatory agents, physical therapy, and local corticosteroid injections. If conservative management fails and symptoms linger, surgical treatment may be warranted. Surgical methods that have been described include excision of the fragment, soft tissue procedures such as lateral retinacular release and vastus lateralis release, and open reduction and internal fixation of the painful fragment. Although good results have been reported with these procedures, many athletes and families are hesitant about surgical treatment. Therefore, we developed a new treatment that can be performed in an outpatient clinic under local anesthetic.
We hypothesized that ultrasound-guided injection and the pie crust technique would be an effective and safe treatment option for symptomatic type III bipartite patella. We examined whether surgical treatment can be avoided in patients who planned surgical treatment with ineffective conservative treatment. The purpose of this study was therefore to investigate patient outcomes after undergoing ultrasound-guided injection and the pie crust technique for the treatment of symptomatic type III bipartite patella, as well as to determine the effectiveness and safety of the novel technique.
Materials and Methods
We performed ultrasound-guided injection and the pie crust technique on 20 knees in 17 male patients (mean [SD] age, 13.1 [1.6] years) between August 2017 and September 2020. The inclusion criteria were as follows: clinically symptomatic type III bipartite patella confirmed by radiography, ultrasonography, and computed tomography; and conventional conservative therapy (eg, restriction of activities, immobilization, administration of nonsteroidal anti-inflammatory agents, and physical therapy) being ineffective for more than 2 months. The exclusion criteria were a history of previous knee surgery and severe degenerative changes of the patellofemoral joint. Patients with fewer than 4 months’ follow-up were also excluded.
Patient Assessment
Patients were clinically assessed by means of the Victorian Institute of Sports Assessment (VISA) score (a scoring system in which higher scores indicate better clinical outcomes) before and 1 week, 1 month, and 3 months after ultrasound-guided injection and the pie crust technique were performed. The occurrence of complications (eg, infection, severe pain, and hematoma) were also investigated during the follow-up period.
Ultrasound-Guided Injection and the Pie Crust Technique
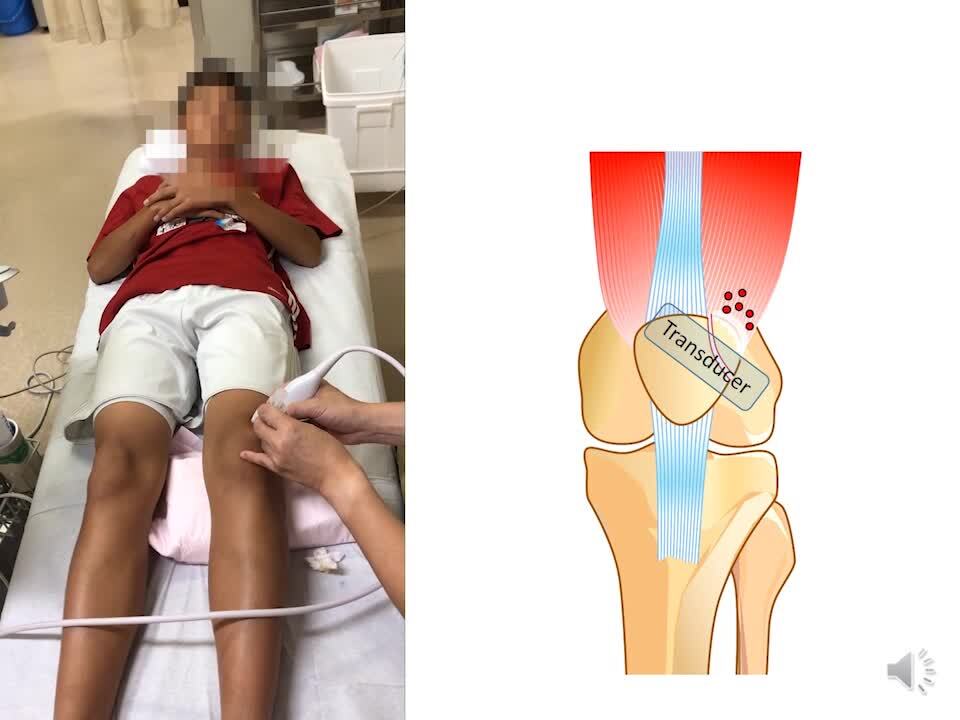
Diagnostic ultrasonography was performed using the SONIMAGE HS-1 ultrasound system (Konica Minolta Healthcare; Tokyo, Japan) with a 18-4 MHz linear transducer. Ultrasound-guided injection and the pie crust technique were performed by a single knee surgeon (J.N.) in an outpatient clinic. The patient was placed in the supine position with the knee slightly flexed to approximately 10° and supported by a pillow. The skin was then cleansed with povidone iodine, after which a 19-mm 27-gauge needle was used to administer 3 mL of 1% lidocaine for local anesthesia. Then, a 25-mm 25-gauge needle was guided into the knee from a lateral approach using a short-axis view of the patella to ensure proper placement just between the patella and free fragment. Once proper placement was established, the surgeon injected 2 mL of 1% lidocaine and 5 mg of triamcinolone acetonide between the patella and free fragment. The surgeon subsequently identified the vastus lateralis tendon and lateral retinaculum contiguous to the accessory fragment and punctured 10 sites from 1 skin puncture with a 38-mm 18-gauge needle to lengthen these structures.
The ultrasonographic procedure was repeated every 2 to 4 weeks according to patients’ wishes. If treatment did not improve their symptoms, accessory fragment extraction was performed under general anesthetic.
Results
The mean (SD) duration from symptom onset to the procedure first being performed was 4.3 (1.9) months, and the procedure was performed an average of 1.9 (1.0) times. Mean (SD) VISA scores were 46.5 (5.0) before treatment, 70.5 (11.4) at 1 week after treatment, 85.4 (14.7) at 1 month after treatment, and 90.3 (16.3) at 3 months after treatment. VISA score improvements from before treatment to 1 week after treatment, and from 1 week to 1 month after treatment, were significant (both P < 0.01), whereas the improvement between 1 and 3 months after treatment was not. Two patients (3 knees) had poor results and could not return to sports, and thus underwent surgical treatment 4 months after the ultrasonographic procedure. However, the other 15 patients were able to fully return to sports a mean (SD) of 4.1 (1.8) weeks after the procedure. There were no complications in any of the patients.
Conclusions
The performance of ultrasound-guided injection and the “pie crust” technique for the lengthening of the vastus lateralis tendon and lateral retinaculum under local anesthetic was found to safely and effectively treat symptomatic type III bipartite patella. This was particularly supported by the significant improvement in the mean VISA scores after treatment and the absence of procedure-related complications. Thus, we recommend the consideration of this novel technique for the treatment of symptomatic bipartite patella before surgical treatment in cases in which other conservative treatment methods are unsuitable.