During a full-day AAOS symposium on orthobiologics in October, more than 400 virtual attendees saw and heard from some 20 content experts who covered this high-interest topic. The symposium, offered free to AAOS members, focused on biologics applied to nonsurgical treatment of osteoarthritis (OA) of the knee, covering the scientific, economic, and regulatory factors involved in this burgeoning field.
David S. Jevsevar, MD, MBA, FAAOS, who co-chaired the symposium with S. Raymond Golish, MD, MBA, PhD, FAAOS, began the session by providing a working definition of “biologics”: a class of therapies that use biological products, by leveraging the body’s natural physiology, to help enhance the body’s healing process. The goal was to explore the science behind the products themselves; the larger issues surrounding biologics, including the FDA’s regulatory role; how payers are or are not reimbursing for biologic therapies; and the role of industry and its interactions with medical professionals, including orthopaedic surgeons and organizations such as AAOS.
In orthopaedic surgery, Dr. Jevsevar said, “These therapies are utilized in both surgical and nonsurgical treatments and have a vast range of clinical applications in spinal fusion, musculoskeletal procedures (anterior cruciate ligament [ACL] repair, rotator cuff surgery, etc.), and articular cartilage restoration, as well as injections for OA of the knee.”
These products come in many categories, noted Dr. Jevsevar, who is also chair of the AAOS Committee on Devices, Biologics, and Technology (DBT). The committee is now working to quantify the number of orthobiologic products currently on the market. “We believe that number to be over 960,” he said, and the products have “many players in the orthopaedic space,” including the physicians who are using them and “the stem cell clinics some of us are concerned about.” He also noted that several professional societies are engaged in the issue of biologics in some way. The DBT Committee and the Academy “want to do our best to work collaboratively with all those societies.”
Dr. Jevsevar indicated that there are probably more than 1,000 stem cell clinics operating in the United States. He said it will be interesting to see how those clinics continue, particularly with direct-to-consumer advertising, given the possibility of greater FDA scrutiny on biologics. In terms of who is offering stem cell and other biologic therapies, “Orthopaedics is slightly greater in involvement, but other specialties are involved,” Dr. Jevsevar said.
“We don’t exactly know how biologics will affect costs,” he continued, noting that there is significant variability in what purveyors are charging and what people are paying for orthobiologics. “Eventually, as the market grows and there is more standardization, there will be a decrease in variability in costs,” he said.
The market for biologics was estimated to be worth $52 billion in 2018, a total that will continue to grow “as efficacy of products coming on to the market is proven, and especially when payers start to pay,” Dr. Jevsevar said, which is generally not the case at present.
He noted that a previous estimate pegged the aggregate cost of knee OA and joint pain at $625 billion, a total that now is probably close to $1 trillion dollars in procedure-related costs. Nonsurgical costs persist up to the time of knee arthroplasty, Dr. Jevsevar said. “People receive a lot of care in the year preceding a knee replacement. With biologics, we want to extend that period and hopefully diminish the need for knee replacement, or at least extend the period a patient is a candidate. One study showed that if we had stricter appropriate use criteria and better nonsurgical treatment, we could have significant impact on the dollars spent. In this study, patients were categorized based on their indications, and, if the researchers were able to extend the indications or timeframe until a patient had a knee replacement, there was a savings of $16,000 over the lifespan of a patient. That’s the future.”
To help orthopaedic surgeons prepare for that future, AAOS has taken a leadership position— researching, making its voice heard in regulatory developments, and disseminating reliable information about biologics to members. The AAOS Biologics Initiative began in 2019, led by the DBT Committee. Its stated focus is to “create evidence-based, unbiased information, thought leadership, position statements, and educational content to help guide orthopaedic surgeons and their patients within this space.”
One of AAOS’ goals is to fund primary research. In partnership with the Orthopaedic Research and Education Foundation, AAOS lets donors earmark funds for AAOS-identified clinical research gaps in orthopaedic surgery. The initiative has yielded a sponsoring grant to conduct primary research on injectable orthobiologics for the treatment of knee OA, with $50,000 from AAOS and $23,000 raised from other donors. The first recipient was Scott Rodeo, MD, FAAOS, for his project titled “Platelet-rich Plasma Treatment of the ACL-injured Knee to Decrease the Risk of PTOA.”
Applications for the second grant funded through that program are now available and will again address injectable orthobiologics. For information, visit www.oref.org/grants-and-awards/grant-programs/grant-program-information#biologics.
The Biologics Dashboard
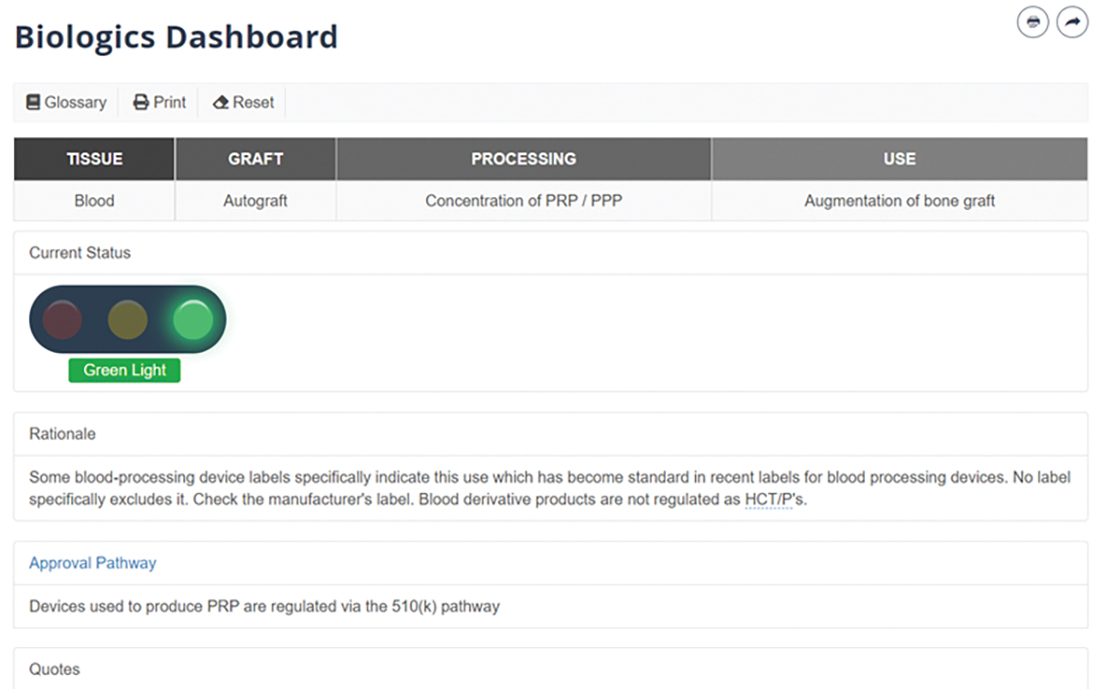
A centerpiece of the AAOS Biologics Initiative is the Biologics Dashboard, an interactive web-based member benefit that uses the “stoplight” paradigm to help orthopaedic surgeons ascertain regulatory status, based directly on cited FDA guidance, for different treatments and indications (see sidebar).
Dr. Golish, a founding member of the DBT Committee, explained that the dashboard “synthesizes numerous sources of information into a smart tool that is easy to query with a few clicks to deepen surgical decision-making in biologics.” He noted that the FDA, industry, and academia provide a wealth of data, but it is difficult to assess the consensus regarding acceptable uses of biologics supported by the relevant regulatory and evidentiary information.
“Where there is no current consensus, the relevant regulatory and evidentiary information is equally critical for informed surgical decision-making and patient consent,” he said. “The dashboard emphasizes regulatory affairs, as these set boundaries for legal clinical use.”
Dr. Golish said the design philosophy of the Biologics Dashboard embraces three principles:
- Serve as an “intelligent” and easy-to-use resource for practicing surgeons.
- Stick close to the source material when there is a clear recommendation.
- When there is no consensus, efficiently synthesize the highest-
quality information from all sources: regulatory status, pivotal trials, and technology reviews.
Other AAOS involvement
Martha M. Murray, MD, FAAOS, also a DBT Committee member, concluded the symposium with an overview of the various ways AAOS and the Biologics Initiative have taken the lead in providing reliable guidance on the use of orthobiologics. In addition to the dashboard, the Academy is also funding primary research, as Dr. Jevsevar detailed, including the full-day symposium itself (supported in part by a grant from Pfizer’s medical grants program) and several events at the 2021 Annual Meeting in San Diego.
At the upcoming 2022 Annual Meeting in Chicago in March, information relating to the DBT Committee and AAOS activity in orthobiologics will be featured at a kiosk in the AAOS Resource Center.
The DBT Committee has also stepped up its issuance of Technology Overviews, which equip members with systematic presentation of relevant published evidence. The overviews offer no recommendations, just summaries of the evidence. Topics addressed so far are:
- WNT2 Pathway Modulators in Knee Osteoarthritis—completed (pilot)
- Concentrated Bone Marrow Aspirate in Knee Osteoarthritis—in review phase
- Platelet-rich Plasma (PRP) in Knee Osteoarthritis—in development“In summary,” Dr. Murray said, “the AAOS Biologics Initiative is going strong.”
A recording of the webinar, “Orthobiologics in Nonsurgical Treatment of Knee Osteoarthritis: Risks and Benefits for Your Patients and Practice,” is available free to members at Webinars On Demand at www.aaos.org/education/courses.
Terry Stanton is the senior medical writer for AAOS Now. He can be reached at tstanton@aaos.org.
New and improved Biologics Dashboard
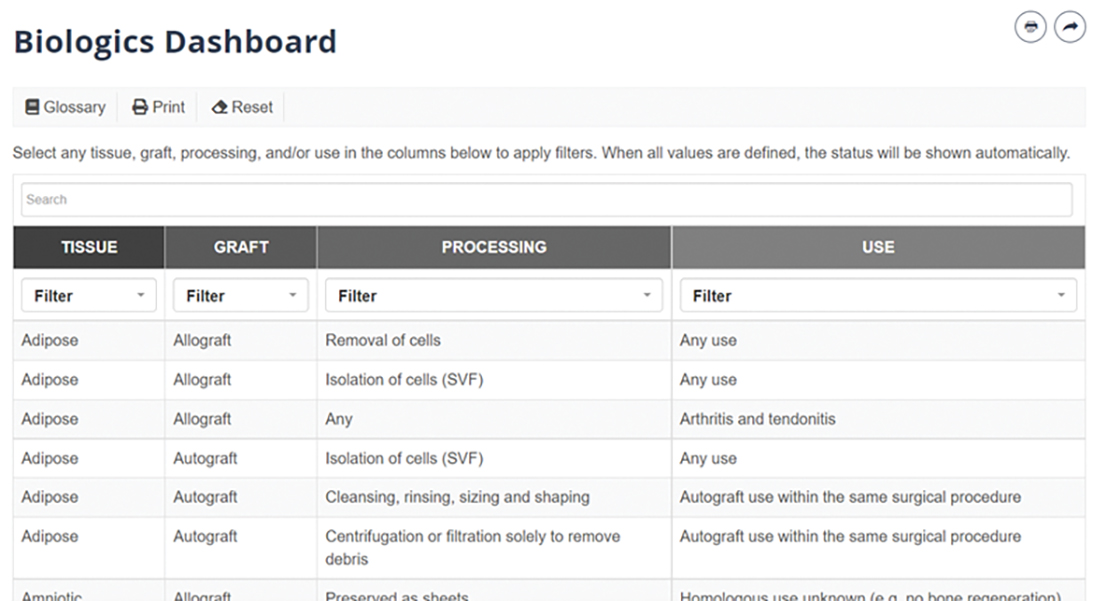
The latest update to the Biologics Dashboard (www.aaos.org/biologicsdashboard) user interface was designed to make it easier for users to find desired scenarios more quickly and with fewer clicks. The full table of possible biologic treatment scenarios is presented on the opening screen. Previously, users had to select values for the fields of tissue, graft, processing, and use, in that order. Now, the updated version allows for nonsequential scenario selection, beginning with any of the four fields (Fig 1). Clicking on a value automatically applies that value as a filter, which lets users quickly and efficiently see which scenarios may be most relevant to their interests. Users can also still search by keyword, which updates the options on the table in real time. Once the table has been narrowed to a single scenario, the output—which includes the green/yellow/red stoplight designation, a rationale for the designation, and direct quotes from federal guidance—automatically loads (Fig. 2)